Acid rain
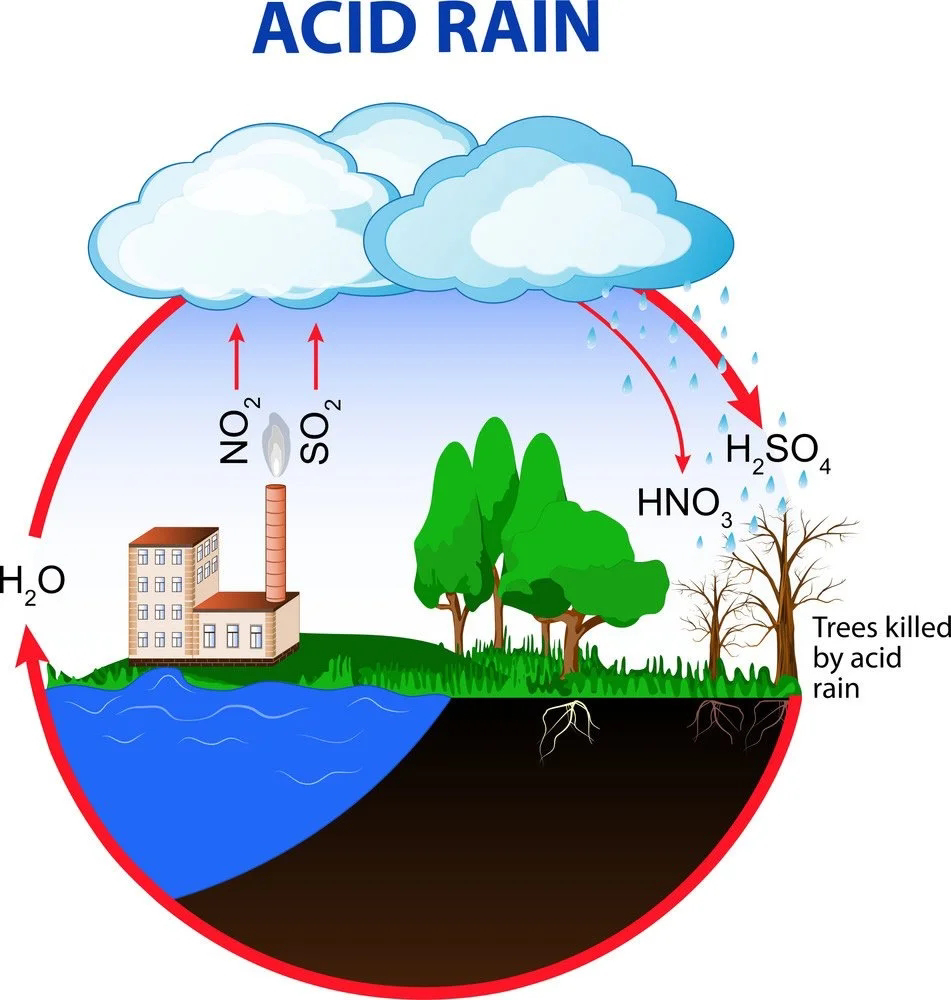
Rain water is actually the purest form of water. However, pollutants such as oxides of nitrogen (N2O, NO2) and sulphur (SO2, SO3) in the air released by factories, burning fossil fuels, eruption of volcanoes etc., dissolve in rainwater and form nitric acid and sulphuric acid which adds up to the acidity of rainwater. Hence, it results in acid rain.
ph of acid rain
Acid rain has pH less than 5.6 whereas pH of pure rainwater is around 5.6 due to dissolution of atmospheric CO2 in it.
Effects of acid rain
Acid rain affects in many ways. Some of the consequences are given below.
*It irritate eyes and skin of human beings.
*It inhibits germination and growth of seedlings.
*It changes the fertility of the soil, destroys plants and aquatic life.
*It causes corrosion of many buildings, bridges etc.
Preventive measures
Acid rain and its effects can be controlled by the following ways.
*Minimising the usage of fossil fuel such as petrol, diesel etc.,
*Using CNG (Compressed Natural Gas).
*Using non - conventional source of energy.
*Proper disposal of the industrial wastes.




கருத்துகள் இல்லை:
கருத்துரையிடுக